Introduction
Until recently, gene knockdown or knockout technologies, such as antisense, ribozyme, and gene knockouts were used to perform loss-of-function studies. However, the post-genomics era calls for high-throughput gene function studies which the former technologies were unable to answer due to poor reproducibility, high cost, and excessive time to results. The advent of siRNA technology has opened up many new possibilities in the field of gene suppression.
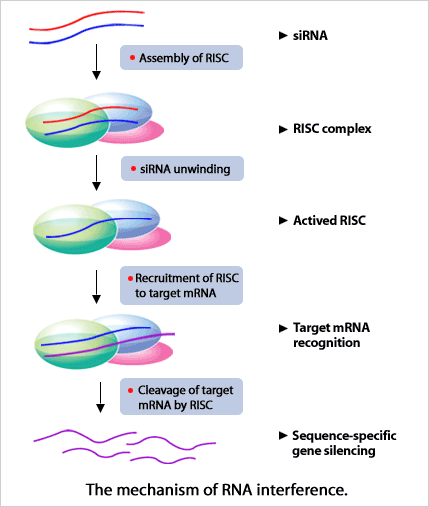
siRNA Mechanism
siRNA is the term for 20 - 25-base pair RNA duplexes, where the two terminal 3'-nucleotides are unpaired (3' overhang). When siRNAs are introduced into cells they combine with a protein complex called the RNA-induced silencing complex (RISC) and are unwound by a helicase. The RISC complex containing single stranded RNA complementary to the target mRNA then recognizes and binds to the target mRNA. After binding the mRNA, the argonaute protein Ago2 cleaves it and complete degradation of the target mRNA is carried out by ribonuclease activity (as a result of the lack of protection by 5' caps or poly (A) tails). This exciting technology is one of the most effective methods for the silencing of specific target genes and is a must for gene function validation studies, drug target validation, and for gene therapy studies. siRNA has the following advantages over other RNAi technology:
• Reduced time and costs: Less screening is needed to obtain highly effective siRNA.
• High efficacy at lower concentration: lower concentrations provide effective gene silencing and minimizes off target effects
• Specificity: siRNA is a highly specific target knockout mechanism based on the natural biological mechanisms of RNAi
Bioneer manufactures high quality and cost-effective siRNA. Every siRNA is produced in an automated high-throughput RNA production system under clean room conditions and undergo rigorous QC tests.
All Bioneer siRNAs are provided as double-stranded siRNA. Each sense siRNA and an antisense RNA are QC'ed by MALDI-TOF. Every annealed siRNA is then QC-tested using PAGE to confirm proper annealing.
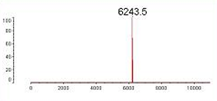
Figure 1. MALDI-TOF mass spectrometry analysis of a custom siRNA. All siRNAs are processed by MALDI-TOF mass spectrometry to ensure its quality.
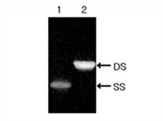
Figure 2. PAGE data of double-stranded custom siRNA. Complementary single-stranded RNA strands were hybridized to form siRNA duplex and analyzed by 15% non-denaturing PAGE.
SS: single-stranded RNA
DS: double-stranded siRNA
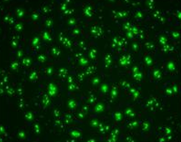
Figure 3. Confocal microscopic image of HeLa cells transfected with FITC-labeled negative control siRNA (Cat No.: SN-1021). The fluorescent cells indicate that the HeLa cells were successfully transfected with the siRNA.
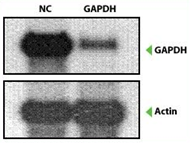
Figure 4. Effects of Human GAPDH Positive Control siRNA. HeLa cells were transfected separately with AccuTarget Human GAPDH Positive Control and Negative Control siRNA using Lipofectamine 2000 (Invitrogen) at a final concentration of 100 nM. Total cellular RNA was isolated from cells 24 hours after transfection and subjected to Northern blot and Real-Time PCR analysis. As can be seen from Fig. 1-B, about 3% GAPDH mRNA remained.
Notice to Purchaser
All siRNA Products: For Research Use Only. Not For Use in Diagnostic Procedures.
Limited License
This product is licensed under European Patents 1144623, 121945 and foreign equivalents from Alnylam Pharmaceuticals, Inc., Cambridge, USA and is provided only for use in academic and commercial research whose purpose is to elucidate gene function, including research to validate potential gene products and pathways for drug discovery and development and to screen non-siRNA based compounds (but excluding the evaluation or characterization of this product as the potential basis for a siRNA-based drug) and not for any other commercial purposes. Information about licenses for commercial use (including discovery and development of siRNA-based drugs) is available from Alnylam Pharmaceuticals, Inc., 300 Third Street, Cambridge, MA 02142, USA.
This product is sold for research use only and is not to be administered to humans or used for medical diagnostics. Buyer acknowledges and agrees that all intellectual property rights in the products (including, without limitation, the siRNA sequences used to create such products) and in any Bioneer technology, intellectual property and know-how used to make or useful for the manufacture or use of the products will at all times remain vested in Bioneer (other than any ownership interest that buyer may have in non-public proprietary target genes supplied by buyer to Bioneer in connection with custom products).
Trademark: AccuTarget is a trademark of Bioneer Corporation.
Synthesis and Order
Q1. How is siRNA synthesized?
The most commonly used method in siRNA synthesizers is the 'phosphite triester' method, developed by Koster. This method builds the phosphodiester backbone of the RNA molecule by using β-cyanoethyl phosphoramidites. (Nucl. Acids Res. 1984, 12, 4539 ; Tetrahedron Lett. 1983, 24,5843) The synthesis begins with a solid support (CPG) with an attached starter nucleoside. A deblocking-coupling-capping-oxidation cycle is repeated to reach the desired length of siRNA molecule. After synthesis, ammonia is used to detach the siRNA from the solid support. The siRNA then undergoes deprotection and TBDMS treatment before being purified. The end result of these processes is pure siRNA. Each synthesized ss-RNA is annealed to produce the final duplexed siRNA form.
Q2. How do I order pre-designed siRNAs?
The
AccuTarget Genome-wide Predesigned siRNAs are designed for Human, Mouse and Rat genomes. The desired target can be searched by Symbol, Gene No, Refseq accession Number or Description. Each query will yield three (3) candidates with the highest predicted efficiency score. You may select the desired quantity at that point. Also, you may select purification type and the guaranteed nmole quantity as well.
Q3. When can I expect delivery?
Delivery depends on the
AccuTarget product that is ordered. Custom siRNA orders can be made and delivered in 10 business days.
AccuTarget Predesigned siRNA can be delivered within 5 days of order receipt. Library products, orders of < 10 96-well plates can be shipped within 3 days of order receipt, and shipping dates for higher quantities above that will have to be discussed on a case-by-case basis.
Q4. Up to how many bases can Bioneer synthesize?
Bioneer offers single strand RNA synthesis service up to 50mers. (not siRNA) The same price is applied up to 30mer siRNA including overhang.
Q5. What form will my order be in?
For Genome-Wide Predesigned siRNAs, Validated siRNAs, siRNA Libraries and Control siRNAs, both the sense and antisense strands are synthesized at equimolar concentrations, verified via MALDI-TOF, then annealed and delivered in duplexed, lyophilized form. You may reconstitute the siRNA with a buffer of your choice or with ultrapure water that we provide with every order. We recommend reconstituting to 100 μM. For Custom siRNA orders, the order is processed by the same method as above. We recommend 50 μM reconstitution for Custom siRNA orders. When ordering Custom siRNA you must select the "Annealing Service" to receive your order in annealed form. If you choose not to use our annealing service, you can use 1X annealing buffer and follow the annealing protocol included with your Custom siRNA order.
Q6. I want to conduct an in vitro experiment. What scale should I choose? What purification should I select?
With a 10 nmole scale siRNA order, you can transfect one hundred (100) 96-well plates at 100 nM per transfection. Unless you are planning to conduct an in vivo experiment, the Bio-RP Purification will yield outstanding results. We recommend HPLC purification for in vivo experimental use.
Q7. Can Bioneer synthesize chimeric RNA?
Yes we can. We have the ability to synthesize chimeric RNA molecules that incorporate dA, dC, dG, and dT DNA bases. We can also incorporate 2'-O-Methyl and 2'-F(rU, rC) into the RNA.
Q8. I would like to order over four (4) siRNA candidates for a single gene. How do I order?
Our Genome-wide predesigned siRNAs provide three (3) candidates per target gene. In order to request more than four (4) candidates, order via Custom siRNA request. The siRNA sequences can be verified only after the order has been submitted. If you would like to compare the sequences with publications or to modify your order, please email us
Q9. The Genome-wide predesigned siRNA didn't work like I expected. What do I do?
When purchasing 3 siRNAs for the same gene, Bioneer guarantees at least an 80% reduction in the target mRNA level for 2 of the 3 siRNAs. If there is not a > 80% reduction in the mRNA level of the target gene, Bioneer will supply 2 siRNAs free of charge* when the customer provides the experiment information.
Q10. Are phosphate groups present on the 5' or 3' ends of the synthesized siRNA?
Unless explicitly stated, the 5' and 3' ends are capped with -OH groups. Therefore, to order 5' phosphate-capped siRNAs, you must request for 5' phosphorylation modification.
Modified siRNA
Q1. What types of modified siRNAs are there?
In order to maximize siRNA applications, modified* siRNAs are available. Although there are many ways to modify siRNAs, the most convenient method is to use phosphoramidites to label siRNAs during synthesis. For a 5' siRNA modification, synthesis is first conducted as normal. At the final stage, however, a labeling phosphoramidite is attached to the 5' end, resulting in a 5' labeled siRNA. 3' labeling is somewhat complicated in that we must start with a labeled phosphoramidite attached to a solid support and build the molecule from there. You can incorporate the label of your choice to the siRNA sequence by using a labeled phosphoramidite at the 5' end of deoxyuridine, therefore allowing for an internal modified siRNA.
The following modifications are offered for our siRNAs.
•
5' Amine - 3' amine modification
• 5' Phosphorylation & 3' phosphorylation
• 5' Thiol - 3' Thiol Modification
• 5' Biotin - Internal Biotin-dT Modification
• 5' Fluorescein - 3' Fluorescein modification
• 3' TAMRA modification
*Certain items may not be available in all countries.
Handling and Storage
Q1. How to I store my siRNAs and how long do they keep?
siRNAs can normally be kept stable at -20°C for over 1 year. The lyophilized form is especially stable and has a long shelf-life. Although solution-dissolved siRNAs can be stable, contamination of the reconstitution solution with RNase will degrade the product. Also, repeated freeze-thaw cycles accelerate the degradation process. Therefore, we recommend that after you receive the siRNA stock, you reconstitute and make several aliquots to avoid such freeze-thawing. Because the phosphodiester bonds of the RNA can break under high pH conditions, we ask you to take caution, and recommend reconstituting in ultrapure water provided.
Q2. How do I reconstitute my siRNA?
If you would like to keep your siRNA in solution, we recommend reconstituting with DEPC-treated water that we provide with your order to maximize stability.
Q3. How to I store my fluorescent dye modified siRNA?
Photobleaching may occur if the fluorescent dye modified siRNA is exposed to light for prolonged periods of time. Therefore we recommend that you store such siRNAs in a dark container and store that container in a dark place.
Purification
Q1. I would like to know how the synthesized siRNA is purified.
To purify newly synthesized siRNA, we have several methods including Bio-RP and HPLC. We select a purification method depending on the end-use of the product:
1. Bio-RP: Phosphoramidites used for siRNA synthesized with the Bio-RP synthesizer have a DMT group protecting the 5' end to prevent accessory reactions from occurring during synthesis. If the DMT group is not removed at the end of the synthesis cycle ('trityl-on' mode), only the desired length N-mer siRNA will have the 5'-DMT group, while the shorter N-1, N-2 etc. synthesis by-products will not have a DMT group attached. Because the DMT group is extremely hydrophobic, it will interface very well with the RP resin. By taking advantage of this fact, and the fact that DMT groups are not present in the N-1, N-2 etc. by-products, we are able to efficiently purify the desired N-mer siRNA. Unless intended for in vivo experiments, the Bio-RP Purification method will yield excellent experimental results.
2. HPLC: For experiments requiring extremely pure siRNAs such as in vivo experiments, Bio-RP purification may be insufficiently pure. In this case, HPLC is commonly used to reach the desired purity level. We use a reversed-phase resin for purification, and this will routinely yield up to 98% purity for 21-mer siRNA molecules. We recommend selecting HPLC purification if you are intending to use the siRNA for in vivo experiment use.
Quantification and Concentration
Q1. What does the O.D. value mean?
Because the amount of synthesized siRNA is extremely small, it is very difficult to quantify by weight. Therefore, we capitalize on the light-absorption properties of nucleic acids to indirectly measure the amount of product. The amount of light absorbed by a given amount of product is expressed as an O.D. (Optical Density) value, and is directly related to the amount of product in solution.
Q2. How do I calculate the amount of siRNA from the O.D. value?
After measuring the amount of 260 nm light absorbed by the siRNA, the following formula is used to calculate the actual amount of siRNA O.D. = εC. The ε is the extinction coefficient which is unique for different materials, and the C stands for the siRNA concentration. If one knows the extinction coefficient and the O.D. value of the siRNA, the concentration can be calculated by substituting those values in the formula above. The ε value for an siRNA molecule is the sum of the extinction coefficient of each base constituting the molecule. The extinction coefficients for each base at 260 nm is are as follows:
rG : 11.5 ml/μmole
rC : 7.2 ml/μmole
rA : 15.4 ml/μmole
rU : 9.9 ml/μmole
Therefore, the extinction coefficient for the entire siRNA molecule can be calculated by multiplying the number of bases by the corresponding extinction coefficient and adding all four products together.
Q3. If my 18-mer siRNA (3G, 4C, 5A, 6U) O.D. value was 0.7, then how much siRNA do I have?
Let's start by calculating the extinction coefficient (εε) of the entire siRNA molecule. ε= 11.5 x 3 + 7.2 x 4 + 15.4 x 5 + 9.9 x 6 = 199.7 (ml/μmole) Therefore, using the O.D. = εC formula, we can calculate the final concentration C to be C = 0.7 /199.7 = 0.0035 (μmole/ml) = 3.5 (nmole/ml). This value is expressed as the 'total nmole' value in your copy of the oligo synthesis report.
Q4. How do I calculate the molecular weight of the synthesized siRNA molecule?
Substitute the number of each base into the following formula: M.W. = (NA x 329.208) + (NC x 305.183) + (NG x 345.207) + (NU x 306.168) - 63.98) + 2.016 NA = Total number of rA NC = Total number of rC NG = Total number of rG NU = Total number of rU
Q5. Can I know how many ng the synthesized product is?
Normally, we will fulfill an order with a guaranteed nmole amount, and the synthesis report will also report the final amount in nmoles. If you must have the ng amount to calculate for an experiment, you can convert from nmole to ng by using the formula below. We make it easy for you by giving you the molecular weight of the siRNA sequence in the report. Molecular Weight (g) X mole (nmole) = Mass of siRNA (ng)
Q6. How do I reach a target concentration?
Each synthesis report gives you a 'volume for 100 pmole/μl' value that stands for the volume of DEPC-treated water you need to add to achieve a 100 pmole/μl concentration. As an example, if the 'volume for 100 pmole/μl' values for two siRNAs are 100 and 200, then you can reconstitute with 100μl and 200 μl of DEPC-treated water, respectively, to reach a 100 pmole/μl concentration.
In other words, the siRNA contained in each tube is:
100 μl x 100 pmole/μl = 10,000 pmole = 10 nmole,
200 μl x 100 pmole/μl = 20,000 pmole = 20 nmole
Although the final use will dictate the concentration of siRNA, we found that for average use, 100 pmole/μl is ideal. That is why we provide you with DEPC-treated water volume for reconstitution to 100 pmole/μl.
Q7. How do I convert between morlarity and moles?
The standard concentration units for oligomers is given in M (mole/L), and the prefixes such as μ (micro), n (nano), p (pico) etc. describe the scope of the unit. The following prefixes are used not only for M, but for other measurement units such as length, mass etc.
10
-1 = deci [d] 10
1 = deca [da]
10
-2 = centi [c] 10
2 = hecto [h]
10
-3 = milli [m] 10
3 = kilo [k]
10
-6 = micro [μ] 10
6 = mega [M]
10
-9 = nano [n] 10
9 = giga [G]
10
-12 = pico [p] 10
12 = tera [T]
10
-15 = femto [f] 10
15 = peta [P]
10
-18 = atto [a] 10
18 = exa [E]
10
-21 = zepto [z] 10
21 = zetta [Z]
10
-24 = yocto [y] 10
24 = yotta [Y]
1 pmole/μl
= 1x10-12 mole / 1x10-6 L
= 1x10-6 mole/L
= 1 μmole/L
= 1 μM
Therefore, μM and pmole/μl are one and the same.
Experiments
Q1. What are some precautions for a siRNA experiment?
First, because not all siRNAs will knock-down the target gene with identical efficiency, you should try 2-3 different sequences to find the best siRNA. Second, to make sure that the knock-down affects downstream protein expression, mRNA levels should also be measured. Thirdly, verify the knock-down phenotype by using another siRNA designed for the same target gene and show that the same phenotype appears.
Q2. This is my first siRNA experiment. How do I set my experimental conditions?
One of the most important factors in a siRNA experiment is the assessment of whether the siRNA gets delivered into the cell. Bioneer offers positive controls that can easily indicate whether the siRNA is being delivered successfully.
Q3. To what cell density should I culture my cells before siRNA transfection?
For siRNA transfection, we recommend that the cell density be approximately 60-70%. (HeLa cell, 6-well plate standard: 1.5 x 10
5 cells/well. We also strongly encourage user optimization of these figures)
Q4. What concentration of siRNA should I use for transfection?
We recommend a starting concentration of 100 nM (100 pmol/well), but strongly advise empirical optimization for the cell line and conditions of your experiment.
Q5. How do I use 10 nmole of siRNA to transfect cells at 100 nM?
This is a source of confusion for many people. In order to transfect a single well with 100 nM siRNA where the transfection volume is 1 ml, you need 100pmole of siRNA. 2 µl of 50 µM (50 pmole/μl) stock siRNA solution in 1 ml will yield 100 pmole. If you were to order 10 nmole of a siRNA, it will be sufficient to transfect 100 wells at 100 nM (100 pmole/ml).
Q6. What transfection reagent do I use?
Because each cell line will have different transfection efficiencies for every transfection agent, we recommend that you select the transfection reagent most suitable for your cell line.
Q7. How do I verify my siRNA transfection efficiency?
You can easily verify the transfection efficiency by transfecting your cells with NC-FITC and observing the cells with a fluorescence microscope. The NC-FITC can also be used as a test reagent to optimize the transfection concentrations of both the siRNA and the transfection reagent.
Q8. Is there a way to know my siRNA's knockdown efficiency beforehand?
The predicted siRNA knockdown efficiency can be seen at the target gene search screen by clicking the siRNA Number. Validated siRNA efficiencies will be presented in blue, while predesigned siRNA efficiencies will be displayed in grey.
Q9. What controls are used for a siRNA experiment?
The AccuTarget Negative Control siRNA is a non-targeting siRNA that has low sequence homology to all known Human, Rat and Mouse sequences. Therefore, it can be used as a convenient negative control for all Human, Rat and Mouse siRNA experiments. The AccuTarget™ Positive Control siRNAs (human) shows great knockdown efficiency for the target gene. We target GAPDH as an endogenous control gene, and also have positive control siRNAs for reporter systems such as GFP and Luciferase.
Q10. How do I verify the siRNA knockdown efficiency?
The siRNA knockdown efficiency can be verified through various techniques including qPCR, Northern Blot, Western Blot etc.
Q11. What are AccuTarget Genome-wide Predesigned siRNAs?
These siRNAs were predesigned for your convenience using our Turbo si-Designer, a siRNA design algorithm co-developed by us and KRIBB (Korea Research Institute of Bioscience & Biotechnology). Using this revolutionary algorithm, we have constructed a high-knockdown efficiency AccuTarget Genome-wide Predesigned siRNA database. As of this writing, our website contains predesigned siRNAs for Human (17,842 genes), Mouse (17,117 genes), and Rat (9,392 genes).
Q12. What are AccuTarget validated siRNAs?
After synthesizing siRNAs using our Turbo si-Designer, a siRNA design algorithm co-developed by us and KRIBB (Korea Research Institute of Bioscience & Biotechnology), AccuTarget Validated siRNAs have been measured for knockdown efficiency using qPCR. We verified that each siRNA shows at least a 70% knockdown efficiency. AccuTarget Validated siRNAs can be ordered with their corresponding real-time qPCR primer sets. Also, if you order 10 or more siRNAs, they will be delivered in flexible siRNA Library (tube or plate type) format.
Q13. Can I order siRNAs argeted for a specific gene?
You may order siRNAs for the target gene of your choice by entering the gene name or Accession number at our website and selecting one of the listed siRNA candidates. You may also order custom designed siRNA if your model organism is not Human, Mouse or Rat.
Q14. What are precautions for handling siRNA?
- Wear gloves and a mask when handling siRNA.
- Always use RNase free tubes and pipette tips.
- Only use DEPC treated water when diluting siRNA.
- Store fluorescence-labeled siRNA in a dark location.