Large scale oligos ranging from mg to gram scales – wide range of modifications available
Bioneer’s production facilities can accommodate large scale oligonucleotide synthesis orders ranging from milligrams to tens of grams of the purest DNA and RNA oligos, including both standard and modified. A large portion of our bulk oligo customers are involved in research requiring antisense oligos, which has opened the door to new approaches in the development of pharmaceuticals and target validation. Some of the frequently used modifications are:
Phosphorothioates and Chimeric Oligos:
General oligonucleotides are subject to rapid degradation by nucleases. Therefore, oligos for antisense application are usually synthesized with a phosphorothioate bond modification to make them resistant to nuclease activity. In phosphorothioates, a sulfur atom replaces a binding oxygen in the oligo phosphate backbone.
2'O-Methyl RNA oligos:
2'O-Methyl RNA increases nuclease stability and affinity of the antisense oligo to the target RNA.
Features and Benefits
-
Free Bio-RP purification: Near HPLC purity at a standard oligo price
-
Broad range of modifications available: If you don’t see it, just ask, we can probably do it
-
Competitive pricing: Great value for your research dollar
OVERVIEW of BIONEER QA/QC
1. Introduction
2. Custom Analytical Service
3. Custom Analytical Service for Antisense Oligonucleotide
4. MALDI-TOF QC System
5. HPLC
6. GC
7. NMR
8. Test of Heavy Metal Content
9. Test of Water Content
10. Bioburden test
11. Endotoxin test
Introduction
At Bioneer, quality control is fundamental to our manufacturing processes and guarantees high quality product. Bioneer's Quality Control for Oligonucleotides can be divided into three distinct areas:
1. Bioneer's Olignucleotide Ordering Systems:
Orders from customers are gathered on a main production server system prior to synthesis. To eliminate re-entry errors, on-line and e-mail orders are recommended. Orders are automatically distributed (batched) to an appropriate synthesizer according to the length of oligo, the type of modification, and users’ plate choice. Every lot to be synthesized is labeled with its own Barcode ID, which is used for identifying the oligonucleotide plate through the synthesis process. Bioneer's Quality Assurance Staff can monitor all procedures from synthesis to aliquoting using our proprietary Automatic Oligonucleotide Production System (AOP System).
2. Automatic MALDI-TOF QC:
Bioneer employs multiple MALDI-TOF mass spectrometers that are fully automated from loading to mass determination. The mass spectrometry data for each sample is automatically inserted into the oligo information sheet. Bioneer is one of the few oligo producers that checks all oligonucleotides (single and high throughput orders) by MALDI-TOF and provides mass data with each oligo, free of charge.

Figure 1. Typical Oligo Datasheet with MALDI-TOF Information
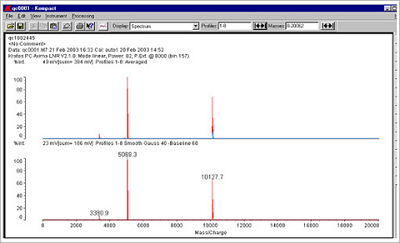

Figure 2. Examples of a typical 30 mer and 23 mer oligo spectrum, in this case employing a Kratos MALDI-TOF system.
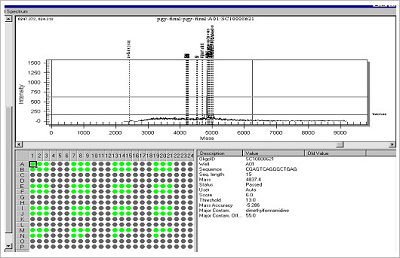
Figure 3. Example of High Throughput Plate QC Analysis.
The mass spectrum and result of 92 (plate) oligonucleotides – Bioneer can also provide “spectrochecked” oligo QC data for users of the Sequenom SNP Analysis System.
3. QC of Large Scale, Antisense and Decoy Oligonucleotides:
The high quality oligonucleotides used in gene therapy and other therapeutic-type applications obviously require more stringent QC. Bioneer has developed specialized QC methods for large scale, antisense and decoy oligonucleotides. These specialized QC procedures comprise HPLC, CE, gel electrophoresis, NMR and additional specific tests such as endotoxin, bioburden, moisture content and etc. Bioneer's wide array of Q.C. procedures satisfies all of our users' specific QC requirements. The array of QC steps available from Bioneer is as follows:
1. Ion-Exchange HPLC analysis
2. Reverse Phase HPLC analysis
3. Capillary Electrophoresis
4. NMR analysis
5. Moisture content analysis
6. Sodium content analysis
7. Heavy metal content analysis
8. Solvent content analysis
9. Endotoxin test
10. Bioburdent test
Bioneer can also supply reports of API stability testing under the contract period. For information on pricing and ordering, as well as additional information on any of Bioneer's specialized QC procedures, please e-mail oligos@bioneer.com.au .
Custom Analytical Service
|
Items |
Tube Price ($) |
Plate Price ($) |
large scale |
|
I-E HPLC |
50.00 |
2.50 (per well) |
50.00 |
|
RP HPLC |
50.00 |
2.50 (per well) |
50.00 |
|
Capillary electrophoresis |
50.00 |
2.50 (per well) |
50.00 |
|
PAGE analysis |
15.00 |
1.00 (per well) |
15.00 |
|
MALDI-TOF analysis, Spectrocheck |
- |
2.00 (per well) |
- |
|
MALDI-TOF analysis, other mass system |
Free |
Free |
Free |
Data will be provided on CD or paper.
Custom Analytical Service for Antisense Oligonucleotide
|
Items |
Tube Price ($) |
Plate Price ($) |
large scale |
|
I-E HPLC |
50.00 |
2.50 (per well) |
50.00 |
|
RP HPLC |
50.00 |
2.50 (per well) |
50.00 |
|
Capillary electrophoresis |
50.00 |
2.50 (per well) |
50.00 |
|
PAGE analysis |
15.00 |
1.00 (per well) |
15.00 |
|
MALDI-TOF analysis, Spectrocheck |
- |
2.00 (per well) |
- |
|
MALDI-TOF analysis, other mass system |
Free |
Free |
Free |
|
NMR analysis |
- |
- |
inquire |
|
Moisture content analysis |
- |
- |
inquire |
|
Metal content analysis |
- |
- |
inquire |
|
Solvent content analysis |
- |
- |
inquire |
|
Endotoxin test (LAL) |
- |
- |
inquire |
|
Bioburden test |
- |
- |
inquire |
MALDI-TOF QC System
The Use of Matrix-Assisted Laser Desorption / Ionization Time of Flight Mass Spectrometry of Synthetic Oligonucleotide QC
At Bioneer MALDI-TOF (Matrix Assisted Laser Desorption-Ionization Time of Flight) is the technology used extensively for failure detection and other problems that cannot be resolved by other methods. Bioneer's fully automated, high throughput QC systems allow the company to provide superior, high quality product superior to that of our competitors. The QC systems installed at Bioneer currently can check the quality of 35,000 synthetic oligonucleotides per day. Each and every oligo is supplied with an oligo data sheet that includes MALDI-TOF mass spectrum.
Interpreting MALDI-TOF Mass QC for Oligonucleotide
A MALDI-TOF mass spectrometer accurately measures molecular weight of a sample. The technique is most useful because it compares the theoretical mass calculated on the basis of oligonucleotide sequence to actual measured data. MALDI-TOF can also be used to check for sequence errors that may occur while inputting sequences. Such a QC method is an absolute requirement for sequence dependent experiments, such as PCR, cloning and sequencing. It can also be used to check whether an oligo has been modified correctly. CE or HPLC analysis cannot be used to check modifications. MALDI-TOF is also used to check for the presence of truncated oligonucleotides and salt contamination.
A MALDI-TOF mass system is most suitable for the QC of oligonucleotides less than 50 bases long. Longer oligonucleotides (> 50 mer) cannot be ionized effectively (100%) by the laser, therefore they cannot be easily detected and therefore will show a poor detection signal that may fail QC. At Bioneer any oligo greater than 50 bases in length are checked for quality by PAGE. PAGE QC data sheets are provided with each oligo > 50 bases.
The Bioneer QC system
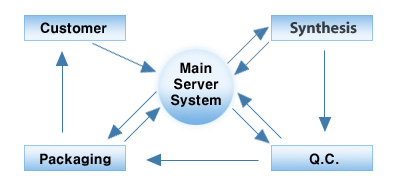
The customer order data is initially saved on the main server system and then transferred to synthesis. Following synthesis, oligonucleotides are spotted on MALDI-TOF mass plates using a proprietary, fully automated, 384-well sample OD quantification/ dispensing robot developed by Bioneer. The MALDI plates are then transferred to QC Division. Any transfer of oligo samples and plates between different divisions and/or equipment requires the bar-code on each sample racks to be checked by a production specialist to confirm the oligo data. Bar coding ensures compliance and allows related divisions to easily retrieve important oligo data from the main server system.
After receiving the oligonucleotides and all the related information, the QC department checks the quality of the oligonucleotides. The mass spectrum of each oligo is saved and the QC program checks whether the oligonucleotides have been synthesized appropriately. Upon completion the final QC data is transferred to the main server.
The Bioneer QC Program is also used to confirm oligo contaminants (including truncated oligonucleotides) present in the MALDI spectra. Another key advantage of Bioneer's QC system is its ability to automatically insert mass spectra (0.6X mass ~ 1.3X mass) of each oligo into the oligo data sheet. Mass spectrum for all ordered oligonucleotides will be provided to customers at no extra charge.
Please note that since the Sequenom Spectrocheck system, which is applicable for some SNP users employs its own program for oligo QC, $100 USD will be charged for every 96 samples if this QC system is required. Mass spectrum data of examined samples can be provided on a CD if required.
MALDI-TOF data is delivered from QC to the main server, and subsequently all the related information is delivered to Packaging so that the correctly synthesized samples will be delivered in the appropriate format as requested by the customer. Bioneer delivers oligonucleotides in a selection of different tubes, 96-well plates, or 384-well plates as per the customers' preferences. After packaged completely, all the oligonucleotides will be shipped by FedEx or UPS, and via their tracking systems, customers may monitor the exact place where the ordered oligonucleotides are in transportation.
In QC, data on all failed samples is automatically returned to synthesis and the re-synthesis of the failed oligonucleotides proceeds whilst QC examines the failed oligo further. This rapid exchange of related information is a key to Bioneer's rapid oligo turnaround time.
HPLC
HPLC Analysis of Oligo Purity
Reverse Phase HPLC
At Bioneer Reverse Phase HPLC is mostly used to QC of intermediates or single stranded DNA produced in the oligo synthesis process. It is a simple QC technique for modified oligo with hydrophobic groups. Reverse Phase is faster and cheaper than Ion Exchange methods and requires less sample.
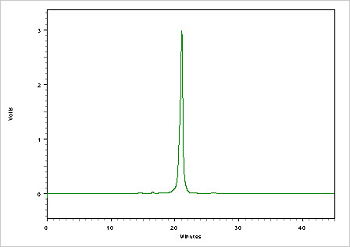
Figure 4. Example of oligo (26 mer) purity analysis using a Reverse Phase (C-18) Column.
Purity Analysis using Ion-exchange Chromatography Method (using Anion-exchange column)
HPLC, equipped with a DIONEX's DNAPac column, is used to QC of oligonucleotides, in particular - Decoy oligonucleotides. The high resolution capability of Ion-exchange can easily separate single stranded DNA and double stranded DNA. At Bioneer Ion-exchange chromatography is commonly used to QC decoy oligonucleotides, and plays a key role in QC confirmation with strict QC standards required for gene therapy.
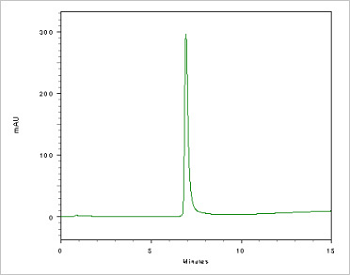
Figure 5. Double stranded DNA (DS DNA) test.
Double stranded oligonucleotides are used for decoy or EMSA experiments. Prior to any experiments, the formation of DS DNA must be checked. For decoy experiments used in the development of new drugs, it is necessary to check the ratio of DS DNA in the decoy. In order to guarantee the medical efficacy, the medicine should be formed in decoy like API from the start of drug development. DS DNA confirmation is an FDA requirement.
GC
Product Purity Test Under Gas Chromatograph
Gas Chromatograph (GC) is used to QC for solvent content in Decoy oligonucleotides and S-oligos used in gene therapy. Prior to administering any oligo based drug to humans it is vitally important to check for the presence of residual organic solvents that may remain after synthesis and purification. Solvent content may compromise efficacy and cause unwanted side effects. The types of residual organic solvents that may be present include acetonitrile, pyridine and toluene etc. Concentrations should be minimally less 0.1%.
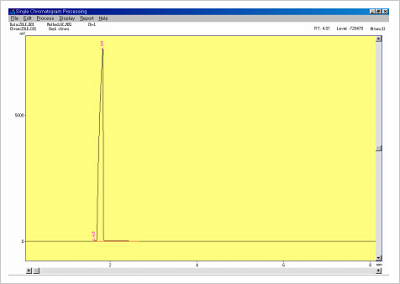
Figure 6. Standard solvent data.
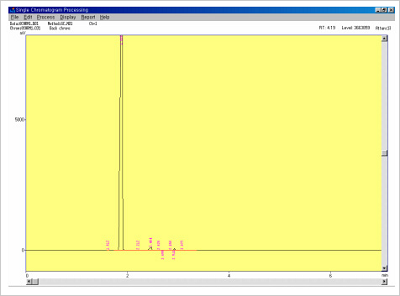
Figure 7.Solvents confirmation data in antisense oligonucleotide
NMR – Spectroscopy
Nuclear Magnetic Resonance (NMR) spectrometer plays a very important role in understanding 3-dimensional structures of molecules. With increasing interests in the structure of biological materials, the use of NMR spectrometer is expanding into new areas, such as drug development, DNA analysis, human genomic and proteomic research and so on. NMR is commonly used to determine physical structure at the molecular level.
At Bioneer NMR is used for 31P-NMR measurement to compare typical frequency values for phosphates present in DNA backbones. By comparing actual frequencies with theoretical it is possible to check the state and purity of phosphates in synthetic oligonucleotides.
Heavy Metal Testing
For antisense and decoy oligonucleotides that are directly injected into animals or humans as medicines in the pre-clinical or clinical phase, it is necessary for check for heavy metal groups that may influence the efficacy or may cause side effects. The types of heavy metals that require QC may differ in each oligo. Inductively Coupled Plasma-Optical Emission Spectrometers (I.C.P), Atomic Absorbance Spectrophotometers (AAS) and I.C.P Mass Spectrometers are routinely employed to QC oligonucleotides for heavy metal groups. Upon request, Bioneer's oligonucleotides can be checked quantitatively/qualitatively for metals such as Lead, Nickel and Fe etc.
Water Content Analysis
Bioneer can also QC oligonucleotides for water content. A Sartorius’ Water Content Measurement instrument (MA-30) is employed to measure water contents that may remain in synthesized antisense oligo following the final drying step of the oligo purification process.
Bioburden Testing
Bioneer confirms the sterility of an aseptic oligo production environment by routinely conducting microbial testing of the water used in the synthesis process and final aliquoting steps. Susceptible areas of potential microbial contamination in the synthesis process and the environment, including operators are also checked periodically. Prevention ensures that the final oligonucleotides will be proven to be safe and free from microbial contaminations.
Endotoxin test
Bioneer also utilizes a Kinetic Chromogenic Analysis (KCA) method to confirm that the oligonucleotides are free of any exothermic materials. Generally exothermic materials present in injectable therapeutics are endotoxins from microbial contamination, especially from Gram negative bacterial contamination and must be avoided.
Kinetic Chromogenic Analysis (KCA) is based on an enzyme linked color reaction (limulus Amoebocyte Lysate reaction). The presence of endotoxins is quantified by measuring color of the reaction against known standards. Many samples can be quantified simultaneously using a standard micro-plate reader. KCA is a fast, cost-effective and short measurement. With such a method, Bioneer only provides oligonucleotides with less than 0.25 EU/ml for therapeutic applications.
OVERVIEW of BIONEER QA/QC
All oligonucleotides synthesized by Bioneer are purified using the Bio-RP purification system. This uses the oligo purification cartridge (OPC) technology and generates oligonucleotides that are 85% pure. This service is available free of cost on all oligonucleotides. We also offer HPLC and PAGE purification.
Bio-RP Purification
The Bio-RP cartridge functions on a basic trityl on selection. In addition to a de-salting, it removes most of the truncated products. This technology yields oligonucleotides that are superior in purity than that obtained by standard ethanol precipitation or gel filtration.
PAGE Purification
PAGE purification is recommended for oligonucleotides between 50-100 bases. This yields oligonucleotides with purity levels over 95% purity. Subsequently yield obtained is lower than other purification techniques.
HPLC Purification
HPLC is recommended for both modified and unmodified oligonucleotides up to 50 bases in length. Our HPLC purifications always start with a trityl on purification, and are often followed by a second full gradient trityl off HPLC. This is our Double RP-HPLC Purification process that gives an extra level of purification. In some cases, the initial trityl-on purification is entirely sufficient and a cartridge is utilized in the subsequent trityl-off oligo processing. This RP-HPLC purification process can yield highly pure material, especially when the oligonucleotide is not complex. When synthesizing compounds that require post-synthetic modification, such as dye labeling, we will perform an additional RP-HPLC purification.