Bioneer’s unique Drug Target & Toxicity Identification Services
Features & Benefits of GPScreen™ Services
-
World's unique & innovative technology
-
Identify any classes of drug targets in genome through genome-wide screening
-
Live cell-based screening
-
High throughput screening
-
Rapid; average 20 days for genome-wide screening
-
Accurate, precise & cost-effective
-
Drug target validation services available in human cells (optional !)
-
Applicable to drug repositioning, natural drug target discovery, toxicity profiling
Ⅰ. Introduction
Precise Drug Target Identification is a first step for improving efficacy, tracing and avoiding side-effects as well as understanding the mode-of-actions of drug candidates. However, until now, effective systematic approaches for the precise drug target identification at genome levels have not been commercially available. Using S. pombe genome-wide heterozygous deletion mutant library, Bioneer has developed a high-throughput genome-wide drug target screening service system (GPScreen™) and made it commercially available for your drug discovery needs. GPScreen™ screening service covers a broad spectrum of drug candidates in whole disease areas from cancers & metabolic diseases to neglected & rare diseases and, therefore, will provide the total solutions for an efficient drug discovery through providing clear-cut answers to problems such as drug-target(s) and toxicity as well as mode-of-actions of drug candidates.
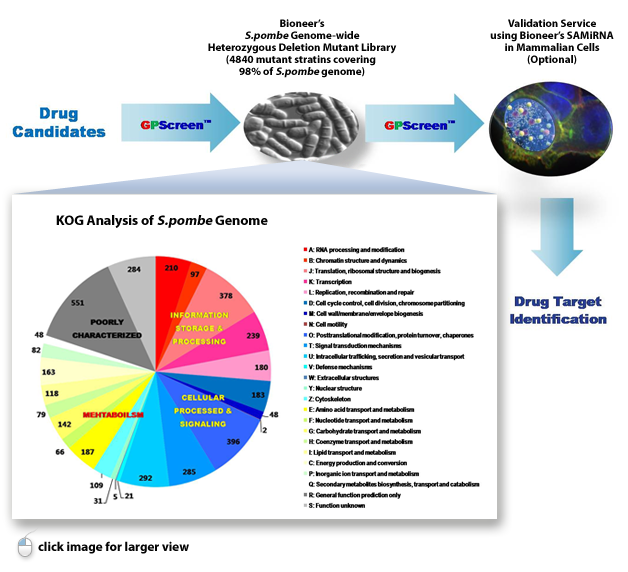
“Bioneer’s GPScreen™ using S. pombe genome-wide heterozygous deletion mutant library has enabled genome-wide screening for drug target identification”
GPScreen™ is based on Bioneer’s unique S. pombe genome-wide deletion mutant library, which was developed by Bioneer and the Korea Research Institute of Bioscience & Biotechnology (KRIBB) in collaboration with Dr. Paul Nurse of Cancer Research Center UK (Nat. Biotech, 28, 617–623 (2010)). It can be used for genetic and chemical screening such as drug target identification, gene expression profiling and synthetic lethal profiling.
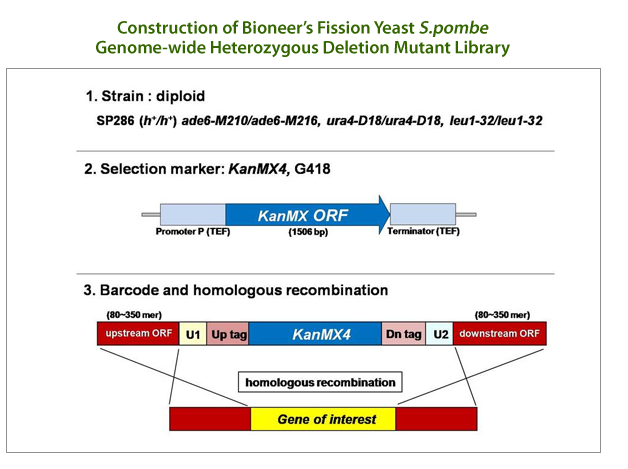 This mutant library comprised of a total of 4,840 heterozygous diploid deletion mutants representing 98.5% genome coverage and one copy of a specific gene in each mutant was deleted by homologous recombination. GPScreen™ exclusively offers S. pombe deletion mutant library as a powerful tool for large-scale genetic functional analysis, identification and verification research of drug targets and for integrated systems research of cell function.
This mutant library comprised of a total of 4,840 heterozygous diploid deletion mutants representing 98.5% genome coverage and one copy of a specific gene in each mutant was deleted by homologous recombination. GPScreen™ exclusively offers S. pombe deletion mutant library as a powerful tool for large-scale genetic functional analysis, identification and verification research of drug targets and for integrated systems research of cell function.
Ⅱ. Principle of GPScreen™
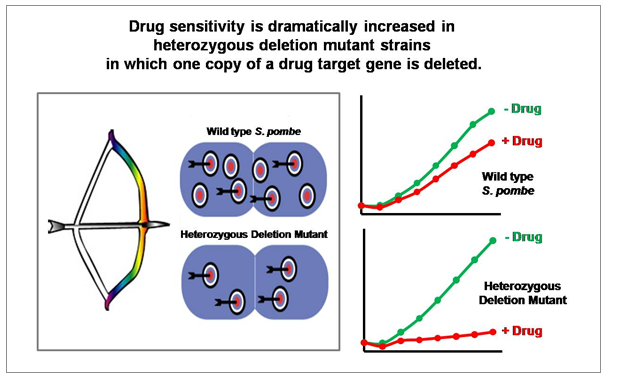
"Drug-induced Haploinsufficiency (DIH)" is a situation in which a strain with a heterozygous gene deletion (producing target proteins to about half of normal levels), result in cells sensitive to a specific drug. DIH occurs when a drug or drug candidate acts on a mutant strain in which the level of target gene protein is lowered from normal to half level, and is considered to be a valuable tool for drug target identification. Previously, a number of reports have provided identifications of drug targets using DIH in budding yeast S. cerevisiae (Baetz K et al., 2004; Lum et al., 2004). However, fission yeast S. pombe is considered a better model of mammalian cells since its cell cycle regulation is closer to that of mammalian cells than that of S. cerevisiae
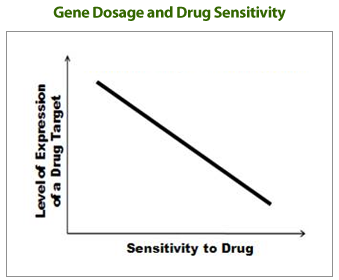
“GPScreen™ utilizes DIH in the fission yeast S. pombe genome-wide heterozygous deletion mutant library to identify the drug target(s).
References
• Baetz K et al. Yeast genome-wide drug-induced haploinsufficiency screen to determine drug mode of action. PNAS. 2004
[More information]
• Lum PY et al. Discovering modes of action for therapeutic compounds using a genome-wide screen of yeast heterozygotes.
Cell. 2004
[More information]
Ⅲ. Performance of GPScreen™
GPScreen™ is a best-in class genome-wide drug target identification system that is exclusively offered by Bioneer Inc. It allows for the clear-cut identification of molecular targets of drugs/ drug candidates (A representative is below).
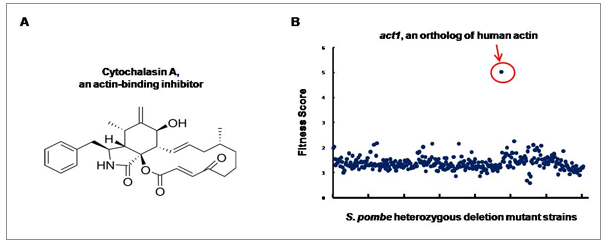
Figure 1. Drug Target Identification using GPScreen™.
In the presence of cytochalasin A (Fig. 1A), a heterozygous deletion mutant of act1 shows decreased growth as a result of a decrease in “functional” Act1 protein. act1 was the only gene in the genome-wide screen to show this effect, demonstrating that act1 is a target of cytochalasin A (Fig. 1B).
Ⅳ. Applications of GPScreen™
 Toxicity profiling and Drug Prioritization
Toxicity profiling and Drug Prioritization
 Drug Repositioning and Enhancement
Drug Repositioning and Enhancement
 Natural Drug Target Discovery
Natural Drug Target Discovery
 Chemogenomic profiling
Chemogenomic profiling
Toxicity profiling using GPScreen™ at early stage of drug development would accommodate 'Drug Prioritization' among drug candidates very efficiently, thereby reducing time and money dramatically in the later stage of drug discovery.
V. Flow chart of GPScreen™ Service
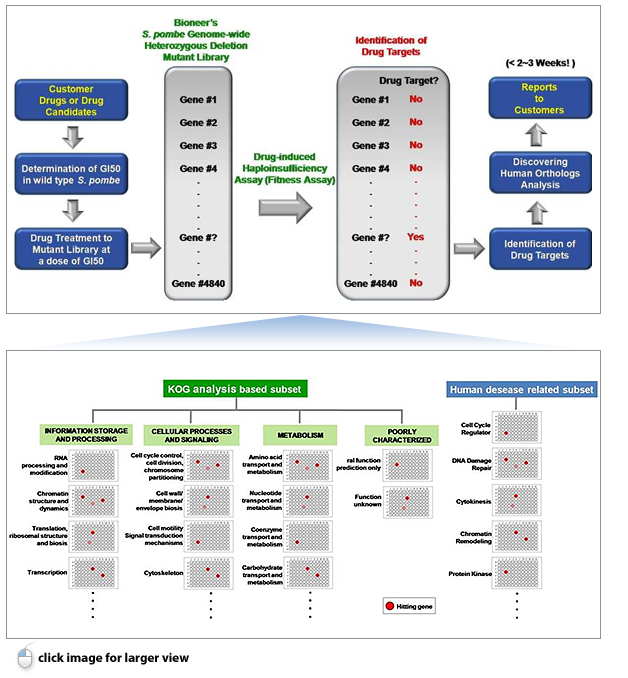
An Innovative Technology for Drug Target Identification using Drug-induced Haploinsufficiency (DIH) in the World’s Unique Fission Yeast S. pombe Genome-wide Heterozygous Deletion Mutant Library
Outline of GPScreen™ Service Process
This service was designed to meet your specific needs fast (results in only 2-3 weeks) at extremely attractive prices. GPScreen™ services is provided with not only genome-wide screening (using 4840 full mutant strains) or as essential gene screening (using 1259 essential gene mutant strains). Furthermore, we can also provide other screening services on specified functional gene groups which are involved in various signaling pathways and/or disease areas (e.g. Cell cycle regulation/cancers) in accordance with your specific needs: By screening only a specified functional subset of the library you can use this service at a reduced price as described below.
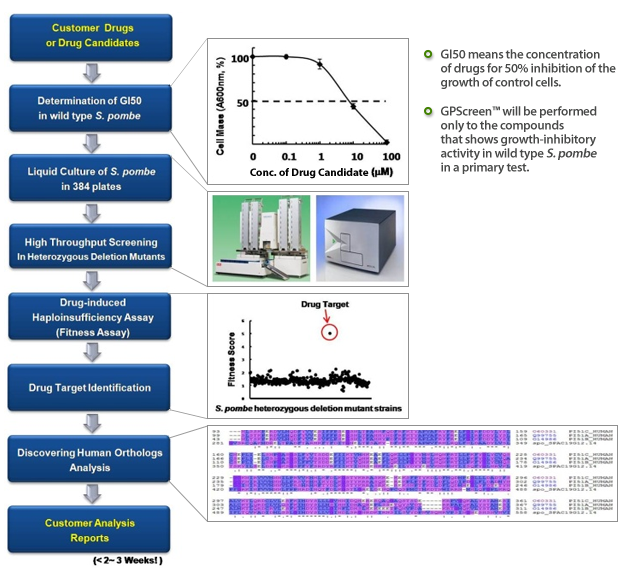
ORTHentication Services exclusively from Bioneer
ORTHentication Services (Ortholog-Authentication services) provide an siRNA-based gene susceptibility test for human orthologs identified through GPScreen™. This allows for validation of the drug targets in human cells. This is done via a method that is similar to Haploinsufficiency screens in yeast (See our GPScreen™): the cells are exposed to the drug and a GI50 is determined for wild type cells. Then they are exposed to the same level of drug after siRNA treatment and subsequent knockdown of the target gene. If the gene in question is being targeted by the drug candidate, then the knockdown of the gene would have a further deleterious effect on cell growth – clearly validating the Human ortholog as a drug target.
Target validation services include
-
Discovering human orthologs of drug target genes identified through GPScreen™ via rigorous bioinformatics analysis
-
Design and synthesis of siRNAs targeting the human orthologs using our exclusive genome-wide human siRNA library (AccuTarget Genome-wide Predesigned siRNA library)
-
Validation of the drug target in human cells by co-treatment of siRNA targeting the corresponding human ortholog (direct mimicking of haploinsufficiency of S. pombe system) followed by the drug treatment
-
Cell biology analysis including proliferation assays in the presence of siRNA and drug; analysis of phenotypic outcome and mode-of-action of the drug
ORTHentication Services are especially well suited for rapid drug repositioning and allow you to fast-track to clinical trial using drug candidates whose toxicity and efficacy tests have been previously completed.
in vivo Validation of ORTHentic (Ortholog-Authentic) genes
Once positively identified, the Human Orthologs can be further validated in an in vivo mouse model. To achieve this, the mouse homolog of the human gene is pulled from our genome-wide mouse siRNA library. This gene is then synthesized into our novel SAMiRNA (Self-Assembled Micelle siRNA) prodrug using a proprietary synthesis method. SAMiRNA is a novel prodrug that is stable in vivo for several days and can be targeted to cell types through targeting molecules on its surface. SAMiRNA also naturally targets Tumors and cancerous tissue through enhanced permeability and retention effects. It has been demonstrated to have no detectable toxicity at therapeutic levels and is the best vehicle for in vivo siRNA delivery available.
SAMiRNA (Self-Assembled-Micelle-interfering-RNA)
One of the major hurdles for the development of RNA interference (RNAi)-based therapeutics is siRNA delivery. SAMiRNA (Self-Assembled-Micelle-interfering-RNA) is a novel class of RNAi molecule developed by Bioneer. The therapeutic potential of SAMiRNA is highlighted by virtue of its negligible toxicity and superb in vivo serum stability as well as its target gene silencing efficacy. These data indicate SAMiRNA’s exceptional therapeutic potential as an RNAi platform for a variety of diseases. Development of SAMiRNA system combines Bioneer’s extensive nucleic acid chemistry and pre-clinical research with our nucleic acid manufacturing and development expertise, which has been providing top-quality DNA oligos and siRNAs worldwide for close to 20 years.
Delivery challenges overcome by SAMiRNA technology
|
Challenges in siRNA delivery |
SAMiRNA NP system |
|
Rapid clearance and degradation in serum |
Improved serum stability |
|
Toxicity of delivery systems |
No detectable liver toxicity or innate immune response |
|
Tissue specificity |
Tumor/tissue targeting capabilities |
|
Silencing potency |
Long lasting in vivo silencing efficiency |
SAMiRNA Custom Services
Bioneer’s SAMiRNA Custom Services provide a wide range of RNAi research services to companies and research institutions worldwide. We offer a complete solution for RNAi (gene silencing) studies and drug discovery. The company’s research and technical support teams ensure the top-quality products and services to meet the unique needs of clients. Our world-class teams have extensive nucleic acid therapeutic manufacturing and development expertise.
Our SAMiRNA Custom Services include:
 Custom siRNA design/synthesis (also offering genome-wide pre-designed mouse and human siRNA library)
Custom siRNA design/synthesis (also offering genome-wide pre-designed mouse and human siRNA library)
 in vitro screen/validation of siRNAs of choice
in vitro screen/validation of siRNAs of choice
 SAMiRNA nanoparticle synthesis
SAMiRNA nanoparticle synthesis
 in vivo siRNA delivery and biodistribution/efficacy test using murine models
in vivo siRNA delivery and biodistribution/efficacy test using murine models
 Quantitation of functional knockdown of target gene by real time qRT-PCR
Quantitation of functional knockdown of target gene by real time qRT-PCR
 And much more
And much more
Features and Benefits
Novel, self-assembling siRNA nanoparticles form with a protective PEG coat and optimal nano-scale size for selective localization in either vascularized tumors through EPR effects, or to targeted tissues through the addition of targeting moieties to the surface of the SAMiRNA.
|
Features |
Benefits |
|
The world's first siRNA prodrug technology |
Stable molecule that is processed in target cell to its active form |
|
Strong intellectual property position around SAMiRNA's unique structure and manufacturing processes |
You're protected working with Bioneer! |
|
in vivo efficacy validated in animal disease models via low dose I.V.injection |
Proven delivery technology |
|
Superb serum stability (PK/PD validated) |
Knockdown efficiency lasts for days in vivo |
|
Extremely low toxicity and cytokine induction |
No detectable toxicity at thera putic levels! |
|
Fully automated solid phase chemical synthesis of siRNA conjugates; advantage of manufacturing & QC processes for large scale production of siRNA drug |
Long half-life and extreme stability in vivo |
|
Powerful siRNA delivery platform technology: Flexibility to in corporate siRNA sequences against any disease target |
Your target-our sequence! |
|
The most cost effective way to prioritize promising gene targets for drug discovery and validate candidate RNAi therapeutics in vivo |
Use our SAMiRNA services today to save time and money |
Novel patent-pending Self-Assembling, siRNA nanoparticles (Figure 1) form with a protective PEG coat and optimal nano-scale size for selective localization in either vascularized tumors through an Enhanced Permeability and Retention (EPR) effects, or to targeted tissues through the addition of targeting moieties to the surface of the SAMiRNA.
-
siRNA Prodrug: highly stable in circulation and siRNA is released and active only when metabolized within the target cells
-
Combined with various targeting moiety, SAMiRNA nanoparticles can target various target organs of interest, for example, liver.
-
Suitable for studying in vivo function of tumor related genes in clinically relevant manner (Figure 2 and 3)
-
The most cost effective way to prioritize promising gene targets for drug discovery and validate candidate RNAi therapeutics in vivo
-
No detectable toxicity or cytokine induction, minimizing off-target effects
A new class of single molecule-based self-assembling Nanoparticles
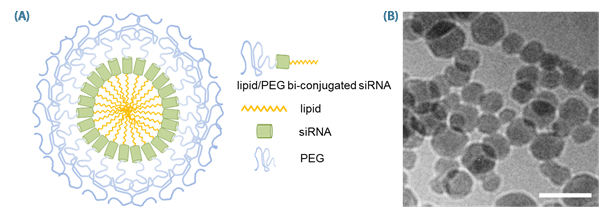
Figure 1. Structure of SAMiRNA Nanoparticle.
(A) Schematic diagram of SAMiRNA (B) Cryo-TEM images of SAM-siRNA Nanoparticles (scale bar = 100 nm).
Long-lasting in vivo efficacy validated in mouse cancer models
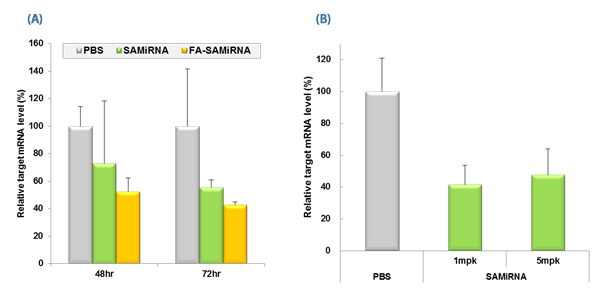 Figure 2. in vivo silencing of target mRNA by SAMiRNA Nanoparticels (NPs).
Figure 2. in vivo silencing of target mRNA by SAMiRNA Nanoparticels (NPs).
(A) Tumor bearing mice were intravenously injected with saline (PBS), SAMiRNA, and tumor-targeting folic acid (FA)-conjugated SAMiRNA Nanoparticles at a single 5mg/kg dose. Mice were sacrificed at denoted time points and targeted survivin mRNA levels in isolated tumor cell mass were subsequently measured by Real-Time PCR. (B) Tumor bearing mice were intravenously injected with saline (PBS) and SAMiRNA NP at a single 1 or 5mg/kg dose. Mice were sacrificed at 72 hr postinjection and targeted survivin mRNA levels in isolated tumor cell mass were subsequently measured using Real-Time qPCR.
Tumor-specific targeting of SAMiRNA NPs in mouse cancer models
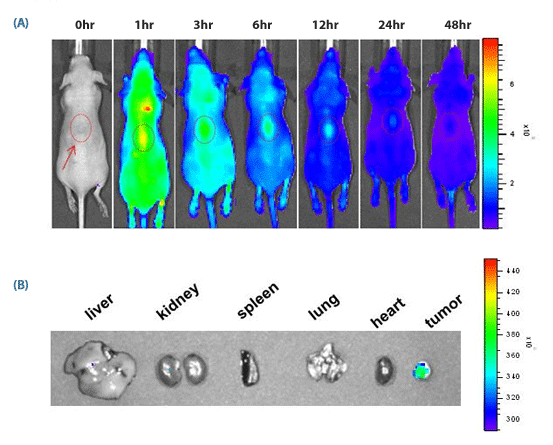 Figure 3. in vivo targeting of SAMiRNA Nanoparticels (NPs) to s.c. grafted tumor.
Figure 3. in vivo targeting of SAMiRNA Nanoparticels (NPs) to s.c. grafted tumor.
(A) Time-dependent
in vivo tumor targeting specificity of Cy5.5-labeled SAMiRNA NPs, delivered via i.v. to tumor-bearing nude mice. (B) Images of various organs extracted from treated mice post-mortem. No fluorescence was detectable outside of the tumor proper.
Ⅰ. How to place a GPScreen™ Service order
To place an order, please follow these steps:
• Download the ordering form. [Download] Note that you will need to fill out one Worksheet/drug compound being tested.
• Complete the order form with your information, product, drug and other relevant info.
• E-mail the completed order form to contact@bioneer.com.au
• Bioneer will confirm order and send service information (service price & period) via e-mail.
• The service can be started the same day the compounds are received.
Ⅱ. How to send us your compounds
* Delivery address:
S. pombe Research Team, Bioneer Corporation
8-11, Munpyeongseoro, Daedeok-gu, Daejeon 306-220, Republic of Korea
(When shipping your compounds, please write your name and organization on your box.)
Ⅲ. Contact Us
For order inquiries and status, please email us at contact@bioneer. com .au
Ⅳ. GPScreen™ Service
GPScreen™ Service provides various screening services to meet the specific needs of customers. Therefore, you can make the best choice among the various services below. After considering the issues and problems of your drug candidates, choose any service you need.
1) Primary Test Service for Determination of Growth-inhibitory Activity (GI50) in Wild Type S. pombe
Primary Test Service will be performed to all compounds requested in advance of GPScreen™
|
Cat.No. |
Primary Test Service |
Cell Type |
Price |
|
GPS-00 |
Primary Test Service in Wild Type S. pombe |
SP286 |
$500.0 /
Compound |
* Price of primary test will be dramatically discounted when the number of compounds is ≥ 2.
2) GPScreen™ Service using S. pombe Genome-wide Mutant Set
|
Cat.No. |
Primary Test Service |
Cell Type |
Price |
|
GPS-00 |
Primary Test Service in Wild Type S. pombe |
SP286 |
$500.0 /
Compound |
3) GPScreen™ Service using S. pombe Essential gene Mutant Set
|
Cat.No. |
Essential Gene Screening Service |
No. of Genes |
Price |
|
GPS-02-ESS |
S. pombe Essential Gene Heterozygous Deletion Mutant Screening Service |
1259 |
Inquire |
4) GPScreen™ Service using KOG analysis-based Functional Group Subsets
|
Cat.No. |
Functional Group-based Subset Services |
No. of Genes |
Price |
|
INFORMATION STORAGE AND PROCESSING |
|
GPS-03K-A |
A: RNA processing and modification Screening Service |
210 |
Inquire |
|
GPS-03K-B |
B: Chromatin structure and dynamics Screening Service |
97 |
Inquire |
|
GPS-03K-J |
J: Translation, ribosomal structure and biosis Screening Service |
378 |
Inquire |
|
GPS-03K-K |
K: Transcription Screening Service |
239 |
Inquire |
|
GPS-03K-L |
L: Replication, recombination and repair Screening Service |
180 |
Inquire |
|
CELLULAR PROCESSES AND SIGNALING |
|
GPS-03K-D |
D: Cell cycle control, cell division, chromosome partitioning Screening Service |
183 |
Inquire |
|
GPS-03K-M |
M: Cell wall/membrane/envelope biosis Screening Service |
48 |
Inquire |
|
GPS-03K-N |
N: Cell motility Service |
2 |
Inquire |
|
GPS-03K-O |
O: Posttranslational modification, protein turnover, chaperones Screening Service |
396 |
Inquire |
|
GPS-03K-T |
T: Signal transduction mechanisms Screening Service |
285 |
Inquire |
|
GPS-03K-U |
U: Intracellular trafficking, secretion, and vesicular transport Screening Service |
292 |
Inquire |
|
GPS-03K-V |
V: Defense mechanisms Screening Service |
21 |
Inquire |
|
GPS-03K-W |
W: Extracellular structures Screening Service |
5 |
Inquire |
|
GPS-03K-Y |
Y: Nuclear structure Screening Service |
31 |
Inquire |
|
GPS-03K-Z |
Z: Cytoskeleton Screening Service |
109 |
Inquire |
|
METABOLISM |
|
GPS-03K-E |
E: Amino acid transport and metabolism Screening Service |
187 |
Inquire |
|
GPS-03K-F |
F: Nucleotide transport and metabolism Screening Service |
66 |
Inquire |
|
GPS-03K-G |
G: Carbohydrate transport and metabolism Screening Service |
142 |
Inquire |
|
GPS-03K-H |
H: Coenzyme transport and metabolism Screening Service |
79 |
Inquire |
|
GPS-03K-I |
I: Lipid transport and metabolism Screening Service |
118 |
Inquire |
|
GPS-03K-C |
C: Energy production and conversion Screening Service |
163 |
Inquire |
|
GPS-03K-P |
P: Inorganic ion transport and metabolism Screening Service |
82 |
Inquire |
|
GPS-03K-Q |
Q: Secondary metabolites biosynthesis, transport and catabolism Screening Service |
48 |
Inquire |
|
POORLY CHARACTERIZED |
|
GPS-03K-R |
R: General function prediction only Screening Service |
551 |
Inquire |
|
GPS-03K-S |
S: Function unknown Screening Service |
284 |
Inquire |
5) GPScreen™ Service using Human Disease-related Subsets
|
Cat.No. |
Human Disease-related Subset Services |
No. of Genes |
Price |
|
GPS-04H-A |
Cell Cycle Regulator Screening Service |
321 |
Inquire |
|
GPS-04H-B |
DNA Damage Repair Screening Service |
376 |
Inquire |
|
GPS-04H-C |
Cytokinesis Screening Service |
124 |
Inquire |
|
GPS-04H-D |
Chromatin Remodeling Screening Service |
257 |
Inquire |
|
GPS-04H-E |
Histone Modification Screening Service |
63 |
Inquire |
|
GPS-04H-F |
Protein Kinase Screening Service |
223 |
Inquire |
|
GPS-04H-G |
GTPase Proteins Screening Service |
90 |
Inquire |
|
GPS-04H-H |
ABC Transporter Screening Service |
31 |
Inquire |
|
GPS-04H-I |
Transcription Factor Screening Service |
362 |
Inquire |
|
GPS-04H-J |
Neurological Disease Screening Service |
19 |
Inquire |
Ⅴ. SAMiRNA in vivo Drug Target Screening
Bioneer’s SAMiRNA Services will provide a rapid e-mail quotation (contact@bioneer.com.au),
comprehensive service packages, and highly competitive prices.
Please let us know the details of your project, so that we can provide you with an accurate quotation and timeline estimate.